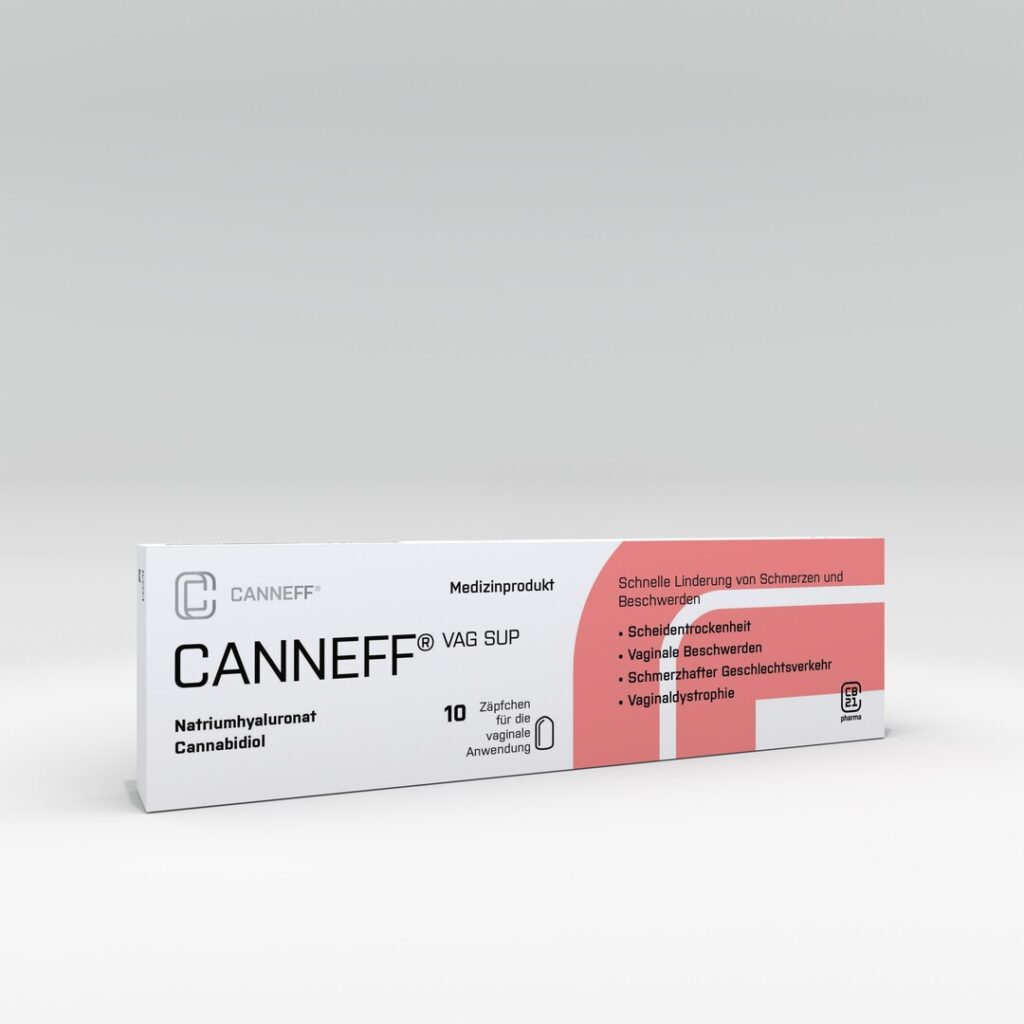
Europe's first over-the-counter CBD medical device
CANNEFF CBD suppositories are used for the natural and hormone-free treatment of pain and discomfort in the intimate area. CANNEFF medical products combine hyaluronic acid and cannabidiol (CBD) in a patented matrix for the effective therapy and treatment of pain and injuries to the vaginal and rectal mucous membranes. CANNEFF CBD suppositories are the first registered medical device with CBD on the European market
Table of contents
Das Geschäft mit legalen Cannabisprodukten floriert nicht nur in Nordamerika.
Der Ausgang der laufenden Zulassung von Hanfextrakt als Novel Food ist angesichts des eskalierenden Konflikts um diesen florierenden Markt noch offen.
Cannabidiol (CBD)
The business with legal cannabis products is not only flourishing in North America. Over the last few years, sales of products containing hemp extracts have also risen steadily in Europe. These mostly contain cannabidiol (CBD) a non-psychotropic cannabinoid. Many rely on it to help them cope with everyday life, which is stressful for many, with more balance. However, industry players continue to navigate choppy waters due to the ambiguity of the current legal framework.
Hemp extracts must not be reduced to medicine
The outcome of the ongoing approval of hemp extract as a Novel Food is still open in light of the escalating conflict surrounding this thriving market. The medical benefits of cannabidiol are being steadily researched and are now considered indisputable for certain indications such as epilepsy. Nevertheless, it remains unclear where and how the line is drawn between medical applications and the functional food/lifestyle market.
Cannabidiol in food products does have merit due to its strong antioxidant and thus cell-protective effects. Here, more social education is needed and politically there is a need for action to carry the benefits of CBD to humans in the best possible way and to draw a clear line of demarcation to the treatment of serious diseases.
The duel for this flourishing market between the pharmaceutical and the food industry increases in intensity. cannhelp shows here possibilities to find the right answers for both areas in the sense of holistic preservation of health and well-being. Philip Schmiedhofer, neuroscientist, medical engineer as well as founder of SBR Development Holding adds „Only with holistic access and knowledge of the endocannabinoid system, the full potential of cannabinoids can be truly utilized.“
CANNEFF CBD Suppositories
cannmedic markets Europe’s first over-the-counter medical product with cannabidiol (CBD) for hormone-free therapy for intimate complaints, such as vaginal dryness, vaginal discomfort or hemorrhoids.
CEO Philip Schmiedhofer adds, „There is an acute need for legally safe CBD products in the EU. As an industry pioneer, we are pleased to be able to market CBD suppositories with legal certainty throughout Europe. In this unclear legal area, a whole new industry has emerged, which definitely deserves more legal clarity in the current proliferation.“
cannhelp, as an active member of the EIHA, is already at an advanced stage of the European approval process for hemp extracts as novel foods. With a focus on high quality and legally compliant CBD products, cannhelp has already been advocating the value of hemp and hemp products in our society for over 9 years.